Key Market Insights
- The landscape of antibody drug conjugates has steadily evolved over the past decade; more than 530 antibody drug conjugates therapy programs are being evaluated by over 140 drug developers, worldwide
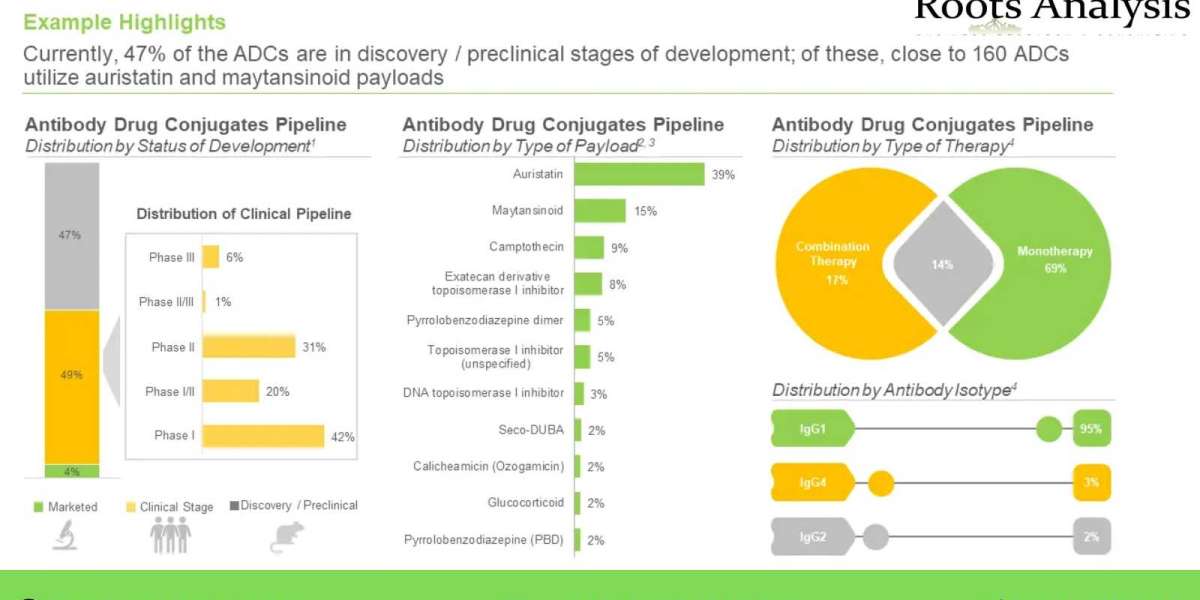
- Currently, 47% of the antibody drug conjugates (ADCs) are in discovery / preclinical stages of development; of these, close to 160 ADCs utilize auristatin and maytansinoid payloads to target a wide array of antigens
- In order to gain a competitive edge, antibody drug conjugate developers are actively conducting multiple clinical trials across different geographies to explore novel targets for the treatment of a wide array of indications
- Since 2010, over 565 clinical trials have been registered to evaluate the safety and efficacy of various antibody drug conjugates; majority of these studies have been conducted across various sites in the US
- Various developers have recently evaluated more than 190 antibody drug conjugates in combination with other therapeutic modalities for the treatment of various oncological disorders
- The growing interest of various stakeholders in this field is evident from the rise in partnership activity over the years; in fact, maximum partnerships were signed in 2022
- Considering the enormous opportunities associated with antibody drug conjugates for the treatment of cancer, several investors have extended funds, worth nearly USD 30 billion, in the last decade
- Several researchers from renowned universities, currently involved in evaluating efficacy and safety of antibody drug conjugates, have emerged as prominent KOLs
- Over the years, the intellectual capital related to the therapeutic applications of ADCs has grown at a commendable pace, with more than 3,330 patents being filed by both industry and non-industry players
- Grants worth over USD 135 million, disbursed across more than 245 instances, have been awarded for research activity related to antibody drug conjugates; nearly 90% of these grants extend a support period of up to 10 years
- Stakeholders are exploring diverse commercialization strategies across different stages of a drug’s launch cycle; for drugs nearing patent expiry, these developers are expected to adopt lifecycle management strategies
- With an objective to keep patients and healthcare professionals abreast with the developments, companies are deploying diverse promotional strategies for their respective products
- Presently, around 35 players, across the globe, claim to have the required capabilities to offer contract manufacturing / conjugation services for antibody drug conjugates; of these, over 10 players are one-stop-shops
- Our proprietary success protocol analysis highlights the impact of over 10 key factors that must be taken into consideration to determine the success of an antibody drug conjugate
- An exclusive cost price analysis offers comprehensive insights on the cost of individual components and likely price of antibody drug conjugates
- With 14 approved antibody drug conjugates and a promising developmental pipeline, the global antibody drug conjugates market is anticipated to witness an annualized growth of nearly 10% over the next decade
- The projected opportunity within this segment is expected to be well distributed across different target disease indication, therapeutic area and key geographical regions
Table of Contents
- PREFACE
1.1. Antibody Drug Conjugate Market Overview
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Key Questions Answered
1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
INTRODUCTION
3.1. Chapter Overview
3.2. Pillars of Cancer Therapy
3.3. Overview of Antibody Drug Conjugates
- Concluding Remarks
- MARKET OVERVIEW
4.1. Chapter Overview
4.2. Antibody Drug Conjugates: Therapies Pipeline
4.3. Antibody Drug Conjugate: List of Therapy Developers
- TARGET COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2. Key Parameters
5.3. Methodology
5.4. Target Competitiveness Analysis: Key Targets of Antibody Drug Conjugates
- COMPANY AND DRUG PROFILES
6.1. Chapter Overview
6.2. ADC Therapeutics
6.2.1. Company Overview
6.2.2. Financial Information
6.2.3. Pipeline Overview
6.2.3.1. Zynlonta
6.2.4. Recent Developments and Future Outlook
6.3. Astellas Pharma
6.4. AstraZeneca
6.5. Byondis
6.6. Daiichi Sankyo
6.7. Genentech (Subsidiary of Roche)
6.8. Gilead Sciences
6.9. ImmunoGen
6.10. Pfizer
6.11. RemeGen
6.12. Seagen
- CLINICAL TRIAL ANALYSIS
7.1. Chapter Overview
7.2. Scope and Methodology
7.3. Antibody Drug Conjugates: Clinical Trial Analysis
- KEY OPINION LEADERS
8.1. Chapter Overview
8.2. Assumption and Key Parameters
8.3. Methodology
8.4. Antibody Drug Conjugates: Key Opinion Leaders
- COMBINATION THERAPIES
9.1. Chapter Overview
9.2. Combination Therapies: History of Development
9.3. Combination Therapies: FDA Guidelines
9.4. Combination Therapies: Antibody Drug Conjugates
- PARTNERSHIPS AND COLLABORATIONS
10.1. Chapter Overview
10.2. Partnership Models
10.3. Antibody Drug Conjugates: List of Partnerships and Collaborations
- FUNDING AND INVESTMENT ANALYSIS
11.1. Chapter Overview
11.2. Types of Funding
11.3. Antibody Drug Conjugates: List of Funding and Investment Analysis
- PATENT ANALYSIS
12.1. Chapter Overview
12.2. Scope and Methodology
12.3. Antibody Drug Conjugates: Patent Analysis
12.4. Antibody Drug Conjugate: Patent Benchmarking Analysis
12.5. Antibody Drug Conjugate: Patent Valuation
12.6. Leading Patents by Number of Citations
- ACADEMIC GRANTS ANALYSIS
13.1. Chapter Overview
13.2. Scope and Methodology
13.3. Antibody Drug Conjugates: Grants Analysis
- KEY COMMMERCIALIZATION STRATEGIES
14.1. Chapter Overview
14.2. Successful Drug Launch Strategy: ROOTS Framework
14.3. Successful Drug Launch Strategy: Product Differentiation
14.4. Common Commercialization Strategies Adopted Across Different Stages of Product Development
14.5. Key Commercialization Strategies Adopted by the Companies Focused on Antibody Drug Conjugates
14.6. Concluding Remarks
- PROMOTIONAL ANALYSIS
15.1. Chapter Overview
15.2. Channels Used for Promotional Campaigns
15.3. Summary of Product Website
15.4. Summary of Patient Support Services and Informative Downloads
15.5. Adcetris: Promotional Analysis
15.6. Besponsa: Promotional Analysis
15.7. Enhertu: Promotional Analysis
15.8. Kadcyla: Promotional Analysis
15.9. Mylotarg: Promotional Analysis
15.10. Polivy: Promotional Analysis
15.11. Trodelvy: Promotional Analysis
- SUCCESS PROTOCOL ANALYSIS
16.1. Chapter Overview
16.2. Antibody Drug Conjugates: Success Protocol Analysis
16.3. Adcetris (Seagen / Takeda Oncology)
16.4. Aidixi (RemeGen)
16.5. Akalux (Rakuten Medical)
16.6. Besponsa (Pfizer / UCB)
16.7. Blenrep (GlaxoSmithKline)
16.8. Elahere (ImmunoGen)
16.9. Enhertu (Daiichi Sankyo / AstraZeneca)
16.10. Kadcyla (Genentech / ImmunoGen)
16.11. Padcev (Seagen / Astellas Pharma)
16.12. Polivy (Genentech)
16.13. Tivdak (Seagen / Genmab)
16.14. Trodelvy (Gilead Sciences)
16.15. Zynlonta (ADC Therapeutics)
16.16. Concluding Remarks
- NOVEL CONJUGATION AND LINKER TECHNOLOGY PLATFORMS
17.1. Chapter Overview
17.2. Antibody Drug Conjugates: Conjugation Technologies
17.3. Antibody Drug Conjugates: List of Conjugation Technologies
17.4. Antibody Drug Conjugates: Linker Technologies
17.5. Antibody Drug Conjugates: List of Linker and Linker-Payload Technologies
17.6. Concluding Remarks
- ASSESSMENT OF NON-CLINICAL DATA FIRST IN HUMAN DOSING
18.1. Chapter Overview
18.2. Antibody Drug Conjugates: Non-Clinical Studies
18.3. ICH S9 Guidelines
18.4. Investigational New Drug (IND)-Enabling Study Designs
18.5. Toxicities in Animal Models
18.6. Prediction of Maximum Tolerated Dosage (MTD) in Humans
18.7. Other Key Considerations for Study Design
- COST PRICE ANALYSIS
19.1. Chapter Overview
19.2. Factors Contributing Towards the High Price of Antibody-Drug Conjugates
19.3. Antibody Drug Conjugates Market: Cost Price Analysis
19.4. Reimbursement Considerations for Antibody-Drug Conjugates
- CASE STUDY 1: CONTRACT MANUFACTURING OF ANTIBODY-DRUG CONJUGATES
20.1. Chapter Overview
20.2. Key Steps in Antibody Drug Conjugate Manufacturing Process
20.3. Technical Challenges Related to Antibody Drug Conjugate Manufacturing
20.4. Challenges Associated with Supply Chain and Method Transfer
20.5. Limitations of In-House Manufacturing
20.6. Investments in Antibody Drug Conjugate Manufacturing Capability Expansions
20.7. Collaborations Established for Antibody-Drug Conjugate Manufacturing
20.8. Growing Demand for Antibody-Drug Conjugate Contract Manufacturing
20.9. CMOs with Linker Manufacturing Capabilities
20.10. CMOs with HPAPI / Cytotoxic Payload Manufacturing Capabilities
20.11. CMOs with Conjugation Capabilities
20.12. Antibody Drug Conjugate One-Stop-Shops
20.13. Increasing Demand for One-Stop-Shops
- CASE STUDY 2: COMPANION DIAGNOSTICS FOR ANTIBODY DRUG CONJUGATES THERAPEUTICS
21.1. Chapter Overview
21.2. Companion Diagnostics for Antibody Drug Conjugates
21.3. Companion Diagnostics For Antibody Therapeutics
21.4. Most Prominent Players: Analysis by Number of Tests
- SWOT ANALYSIS
22.1. Chapter Overview
22.2. Strengths
22.3. Weaknesses
22.4. Opportunities
22.5. Threats
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
23.1. Chapter Overview
23.2. Forecast Methodology and Key Assumptions
23.3. Global Antibody Drug Conjugates Market, 2023-2035
23.4. Antibody Drug Conjugates Market: Product-wise Sales Forecast, 2023-2035
- EXECUTIVE INSIGHTS
24.1. Chapter Overview
24.2. Oxford Biotherapeutics
24.3. Angiex
24.4. Syndivia
24.5. BSP Pharmaceuticals
24.6. PolyTherics (an Abzena company)
24.7. CureMeta
24.8. CytomX Therapeutics
24.9. NBE-Therapeutics
24.10. Cerbios-Pharma
24.11. Eisai
24.12. AbTis
24.13. AmbrX
24.14. Synaffix
24.15. Pierre Fabre
24.16. Catalent Pharma Solutions
24.17. Lonza
24.18. Piramal Healthcare
24.19. Ajinomoto Bio-Pharma Services
24.20. Cardiff University
24.21. Anonymous, Director, Business Development, Leading CMO
24.22. Anonymous, Chief Executive Officer, Leading CMO
- CONCLUSION
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/view_document/antibody-drug-conjugates-market/270.html
Learn from experts: do you know about these emerging industry trends?
Novel Cell Cytometers: Need of the Hour
Patient Recruitment – Key Determinant of Successful Clinical Trials
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415